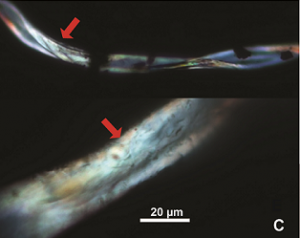
Study finds cotton fibers dyed 7,000 years ago in the Jordan Valley
In the News Cotton fibers from Tel Tsaf. | Credit: Scientific team at Tel TsafThe discovery made by an international team led by Prof. Danny Rosenberg (School

A new study published recently in the journal Current Biology suggested that during the course of evolution, most animal species on Earth lost the ability to synthesize cholesterol autonomously. Cholesterol is a lipid molecule essential for key aspects of animal life including growth and reproduction. For instance, the steroid hormones that manifest the sexual identity of humans are made from cholesterol. However, impaired cholesterol homeostasis has consequences detrimental to human health including a higher risk for cardiovascular disease.
A team of researchers led by Dr. Amir Sapir from the Department of Biology and Environmentin collaboration with Prop. Yoram Gercham, has identified a unique genetic signature in animals that do not synthesize cholesterol such as the fruit fly and the soil worm C. elegans. The research team used this signature to study the evolution of cholesterol synthesis across the animal kingdom. The analysis suggests that cholesterol synthesis evolved at the base of the animal kingdom and conserved in some animals, including mammals. Surprisingly, the analysis also predicts that more than 80% of the animal species on Earth lost the ability to make cholesterol, including central groups such as cnidarian (e.g. jellyfishes and corals), nematodes, and arthropods (e.g. insects and spiders).
This discovery raised a fundamental question: How do animals that lost the ability to synthesize cholesterol survive? To address this question the team used a multidisciplinary approach combining genetic, biochemical, and metabolic methods leading to a finding of a novel metabolic pathway used by these animals for the conversion of plant and fungal sterols to cholesterol. This pathway relies on the repurposing of enzymes whose original function was to synthesize cholesterol, for the conversion of plant and fungal sterols into cholesterol.
This study presents a molecular rewiring of widespread food webs during the course of evolution. The findings have global implications on our understanding of the cross-talk between animals and other organisms, i.e. fungi and plants. “The implications of our findings are widespread and self-evident in our daily life, for example, the molting of a butterfly, the stinging of a jellyfish, diversity of coral reefs, and the honey of the bees all rely on the conversion of plant and fungal sterols to cholesterol using the pathway we identified,” says Dr. Sapir. Furthermore, this study opens new avenues for more ecological and metabolic questions, for example, why and when did most animals on Earth lose the ability to synthesize cholesterol? Do vertebrates like cows and sheep that feed on grass have similar ability to convert plant sterols to cholesterol? Why do obligate predators like lions and cheetahs, with a diet composed of a high level of cholesterol, still retain the cholesterol synthesis pathway? Elucidating these questions can also shed light on certain aspects of cholesterol metabolism in human health and disease – what are the consequences of a cholesterol-free vegan diet versus cholesterol-rich keto or paleo diets? To address these new questions, Dr. Sapir and his team are using cutting-edge approaches of genetic bioinformatics and biochemistry of metabolism. For example, currently running in their laboratory is an experiment of evolutionary engineering in which the researchers are trying to restore the ability to synthesize cholesterol to an animal that lost this property in the course of evolution by expressing cholesterol-synthesizing enzymes from humans in the fly Drosophila in order to try restoring cholesterol synthesis. “We expect experiments such as these will lay the foundation for better understanding of how evolution shaped the metabolic network of the living world, and will open the gate for future studies about the genetic control of cholesterol metabolism in the human brain and cardiovascular system.

In the News Cotton fibers from Tel Tsaf. | Credit: Scientific team at Tel TsafThe discovery made by an international team led by Prof. Danny Rosenberg (School

A study reviewing 220,000 years of Dead Sea geology predicts the occurrence of a major seismic event to hit the region in the next few

In the News Based on new data extracted from DNA samples of ancient rodents, a team of Israeli and international researchers led by Dr. Ignacio
© University of Haifa Division of External Relations